Vaccine Overview
Covaxin™ (BBV152) An Investigational Whole-Virus Inactivated COVID-19 Vaccine*
Key Findings: Phase 3 Clinical Trial Involving 25,800 subjects
Protective Effect
78% All Disease
65% Delta Variant
AE Profile
12% Adverse Events**
<0.5% Serious Adverse Events**
**Placebo group
Covaxin (BBV152) Characteristics
- Both humoral & cellular responses generated against multiple viral proteins
Induces a Th1 response (cell-mediated immunity) - Technology platform used to produce Polio, Influenza and Rabies vaccines
- Antigen: 6μg inactivated SARS-CoV-2 (NIV-2020-770)
- Adjuvant: Algel+IMDG (TLR7/8)1
- Premixed and stable at standard refrigerated
conditions (2°-8° C), with a 2 year shelf life
Administration: 2 doses, 28 days apart
Role of Adjuvant in Immune Response 2,3
Whole virion inactivated vaccines are typically formulated with Alum to induce an antibody response. These vaccines have demonstrated a primarily Th2 humoral immune response with limited cellular immunity. Emerging evidence suggests both antibody (humoral) and primarily T-cell mediated immunity are necessary for effective COVID-19 protection. Covaxin (BBV152) is formulated with Alum + IMDG, a novel adjuvant that has been shown to illicit a strong Th1 biased antibody response and provide BOTH humoral antibody and cellular (T-cell) immune responses. Covaxin (BBV152) is the first whole virion COVID-19 vaccine reported to induce a Th1 biased response.
Covaxin is an Investigational Whole Virus Inactivated Vaccine
Covaxin™ (BBV152)
(multiple targets)
mRNA and Adenovirus-Based Vaccines
(only one target)
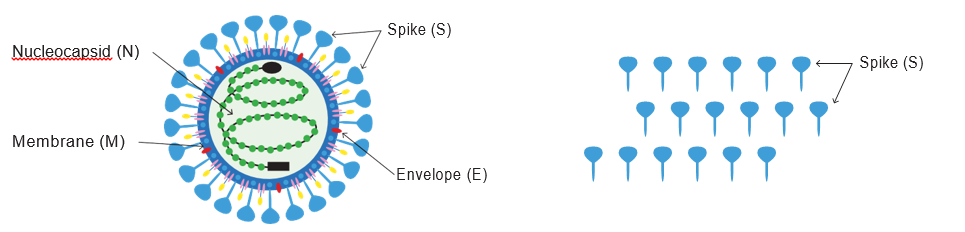
1. Novel adjuvant developed in collaboration with NIH/NIAID
Fold Change in Neutralization Titer— by Sera Type
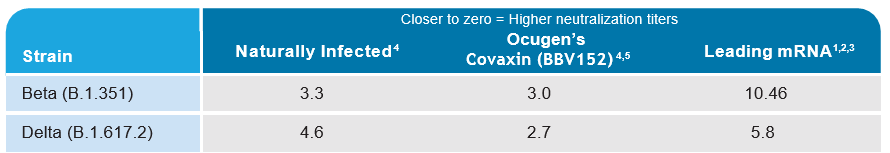
1. https://www.biorxiv.org/content/10.1101/2021.05.31.445871v1
2. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01290-3/fulltext
3. https://www.biorxiv.org/content/10.1101/2021.05.09.443299v1
4. https://www.biorxiv.org/content/10.1101/2021.06.05.447177v1
5. https://www.biorxiv.org/content/10.1101/2021.04.23.441101v1
Rare Adverse Event Profile
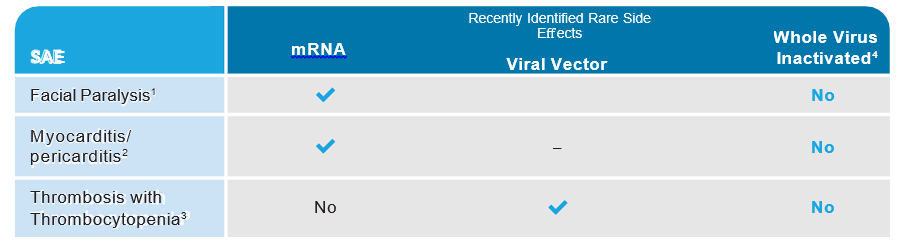
This table is for informational purposes and not meant for direct comparison. The data are from pharmacovigilance and not a comparative clinical study.
Covaxin’s (BBV152) Manufacturing Process:
The Vero Platform
Transport, Storage and Shelf Life:
Designed to Fit Within Existing Stockpile,
Retail Pharmacy and Physician Office Models
10 dose vial
Store/ship 2°-8° C
2 year shelf life
3 month stability at room temperature

Investigational Vaccine
For reference only
Vaccine Overview
Our vaccine works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus.


Vaccine Overview
Our vaccine works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus.

Vaccine Overview
Our vaccine works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus.

